Clinical study
The main goal of the MELISSA project is to test the new MELISSA app that gives people living with diabetes daily, personalised advice on insulin and diabetes management. This testing takes place with the support and close supervision of medical staff.
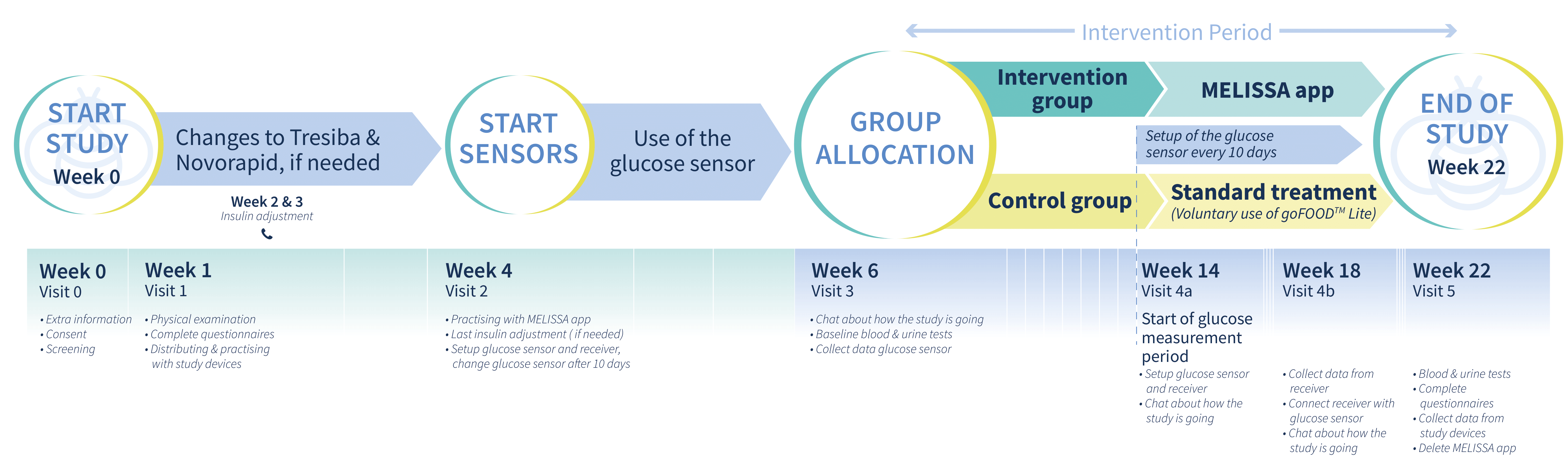
The study will take place at five clinical sites in four European countries, these are The Netherlands, Denmark, Germany and Greece. The clinical study will include approximately 490 people with type 1 and type 2 diabetes on multiple daily injections of insulin. The total duration of the study will be 22 weeks.
Today, there is no smartphone app with an artificial intelligence-based insulin and carb calculator available for the wider diabetes community. The MELISSA project aims to test how well the app works in people living with diabetes. Afterwards, this project will seek approval from health authorities in European Union for widespread use in diabetes care.
An overview of the clinical study:
Participants needed for the MELISSA clinical study
Participate in the MELISSA project by using the app, possibly improving your own diabetes care, and help us improve diabetes care for others.
What is the MELISSA study about?
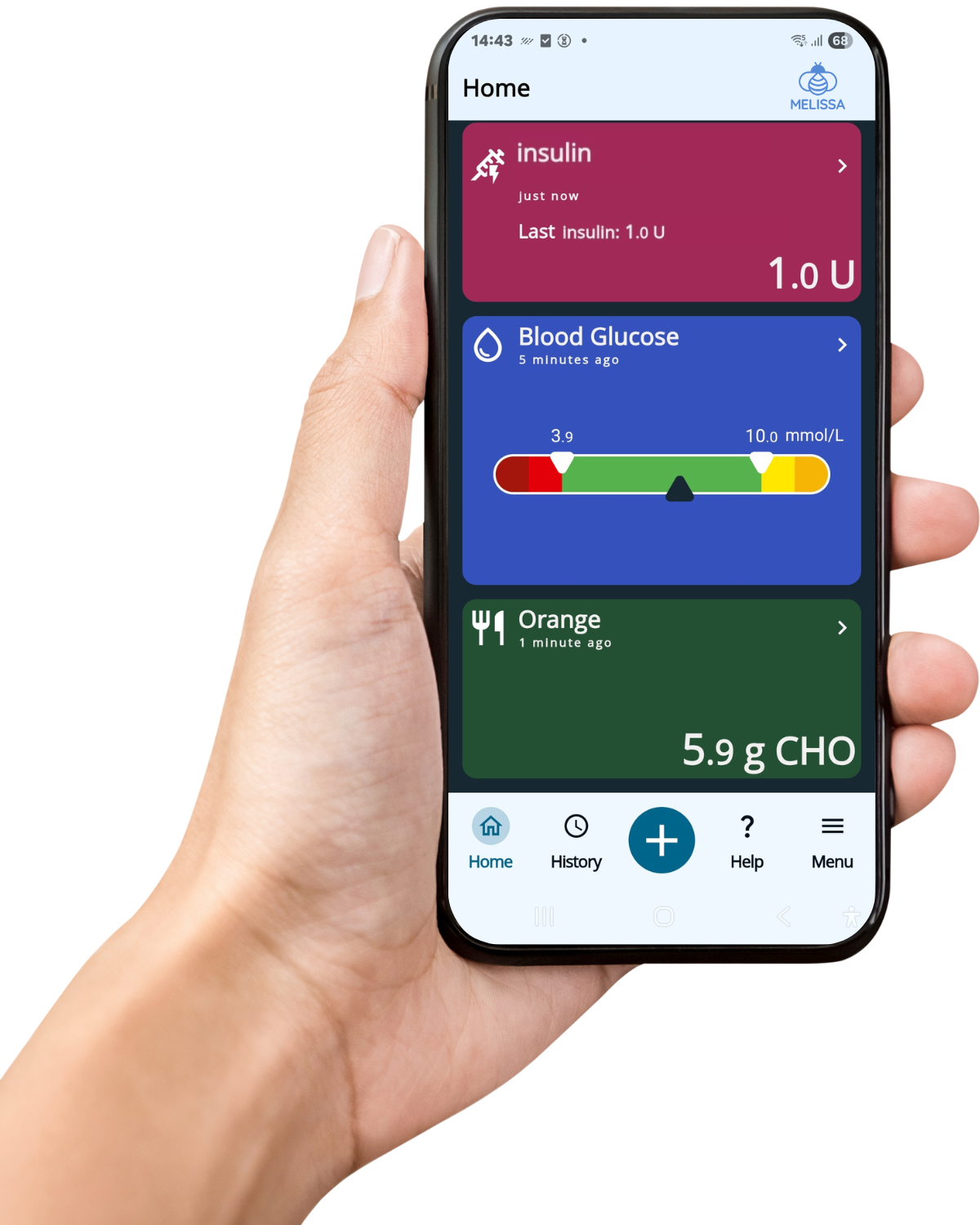
The MELISSA app contains an artificial intelligence-based insulin and nutrient calculator – including estimations on calories, carbohydrates, fat and protein – created to assist diabetes self-care. The MELISSA app can be used with a continuous glucose monitor (CGM) or glucose meter, insulin pen and automatic carb counting to provide personalised insulin advice. The MELISSA project strives to improve glucose control, support your diabetes self-care and improve quality of life.
Why participate?
- Potentially enhance your daily glucose control
- Compensation: not only will you be at the forefront of diabetes management technology, but you will also receive a free activity tracker and reimbursement for your travel expenses.
- Be part of something bigger: Participate in the first study of its kind in Europe, aimed at revolutionising diabetes self-management through artificial intelligence.
Who can participate?
- People living with type 1 or 2 diabetes
- On multiple daily injections with insulin for more than 1 year
- 18 years* or older
*16 years or older at PAK (Greece)
What your participation involves.
The study will last for 22 weeks. You will be invited for a screening visit, a number of in-person visits and 2 phone call visits. Throughout the study period, we will ask the following of you:
- Completing questionnaires on topics regarding your diabetes and skills regarding your diabetes self-care
- Using the MELISSA app, following insulin advice and monitoring your glucose levels, insulin, food intake, and daily events
- Wearing a physical activity tracker (voluntary)
- Wearing a blinded (meaning that you cannot see your glucose data) continuous glucose monitor (CGM). Please note that you will still use your own continuous glucose monitor or glucose meter to check your glucose levels during the trial.
Clinical Sites
Interested in being part of the MELISSA project? Please click on your clinical site for contact details and more information.
Denmark, Nordsjællands Hospital (NOH) & Steno Diabetescenter Copenhagen (SDCC)
Interested in being part of the MELISSA project? Please contact us for more information:
Email: melissa-diabetes.nordsjaellands-hospital@regionh.dk
Phone: + 45 30547323
Germany, Otto-von-Guericke-Universitaet Magdeburg (OVGU)
Following soon.
Greece, National and Kapodistrian University of Athens (NKUA)
Following soon.
Greece, Geniko Nosokomeio Paidon Athinon Panagiotoy Kai Aglaias Kyriakoy (PAK)
Following soon.
The Netherlands, Maastricht University (MUMC+)
Interested in being part of the MELISSA project? Please contact us for more information:
Email: Melissa-intmed@maastrichtuniversity.nl
Phone: + 31 433884030